
Exhibit 99.2
| April 10, 2021 |  |
Greenwich LifeSciences Presents Immune Response Phase IIb Poster, Published Today at AACR 2021, Showing Peak Immunity after 6 Months of GP2 Treatment, Resulting in 100% Disease Free Survival from Recurring Breast Cancer
| ● | Poster published today at the 2021 American Association for Cancer Research (AACR) Annual Meeting shows the GP2 final 5 year immune response data from the Phase IIb clinical trial, concluding that GP2 immunotherapy generated GP2-specific immune responses leading to promising clinical benefit, thus supporting GP2’s mechanism of action. |
| ● | The corresponding abstract can be viewed at the bottom of this press release and the full poster, Figures 1-3, and an audio track of the poster can be accessed or downloaded from the Clinical Trial tab of the Company website: https://greenwichlifesciences.com/clinical-trials/#Phase-IIb-AACR. The audio track, given by Dr. Jaye Thompson, Vice President of Clinical and Regulatory Affairs, can be directly played here: https://greenwichlifesciences.com/wp-content/uploads/2021/04/Thompson-Ph2-AACR-2021.m4a. | |
| ● | Immune responses to GP2 were measured over time using a CD8 T cell dimer binding assay (Dimer Binding Assay) and delayed-type-hypersensitivity (DTH) skin tests. The Dimer Binding Assay detects the percentage of GP2 specific killer T cells that can kill recurring cancer cells. The DTH skin test measures the diameter of the skin immune response to GP2 in millimeters 48-72 hours after injection of GP2 without GM-CSF. | |
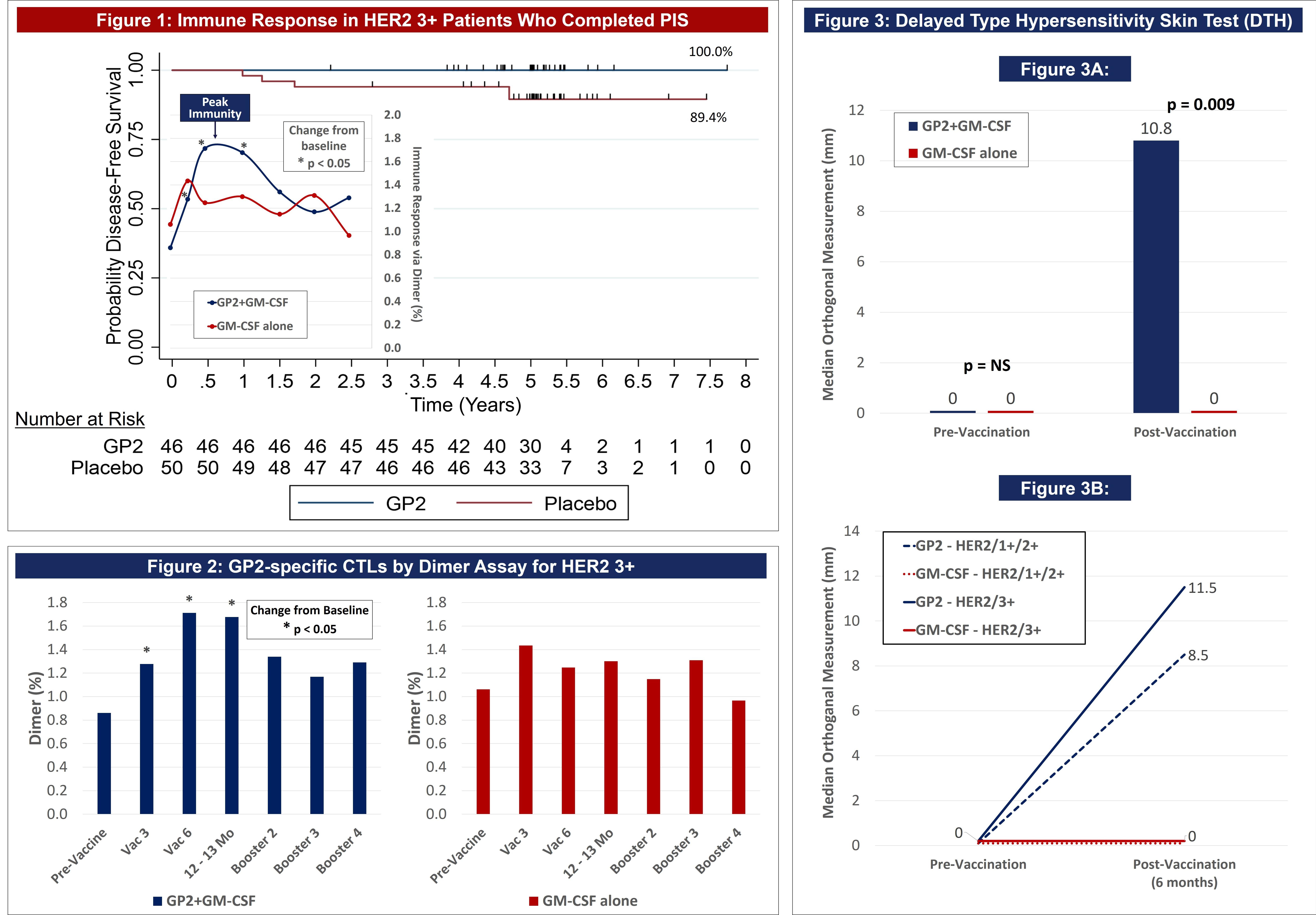
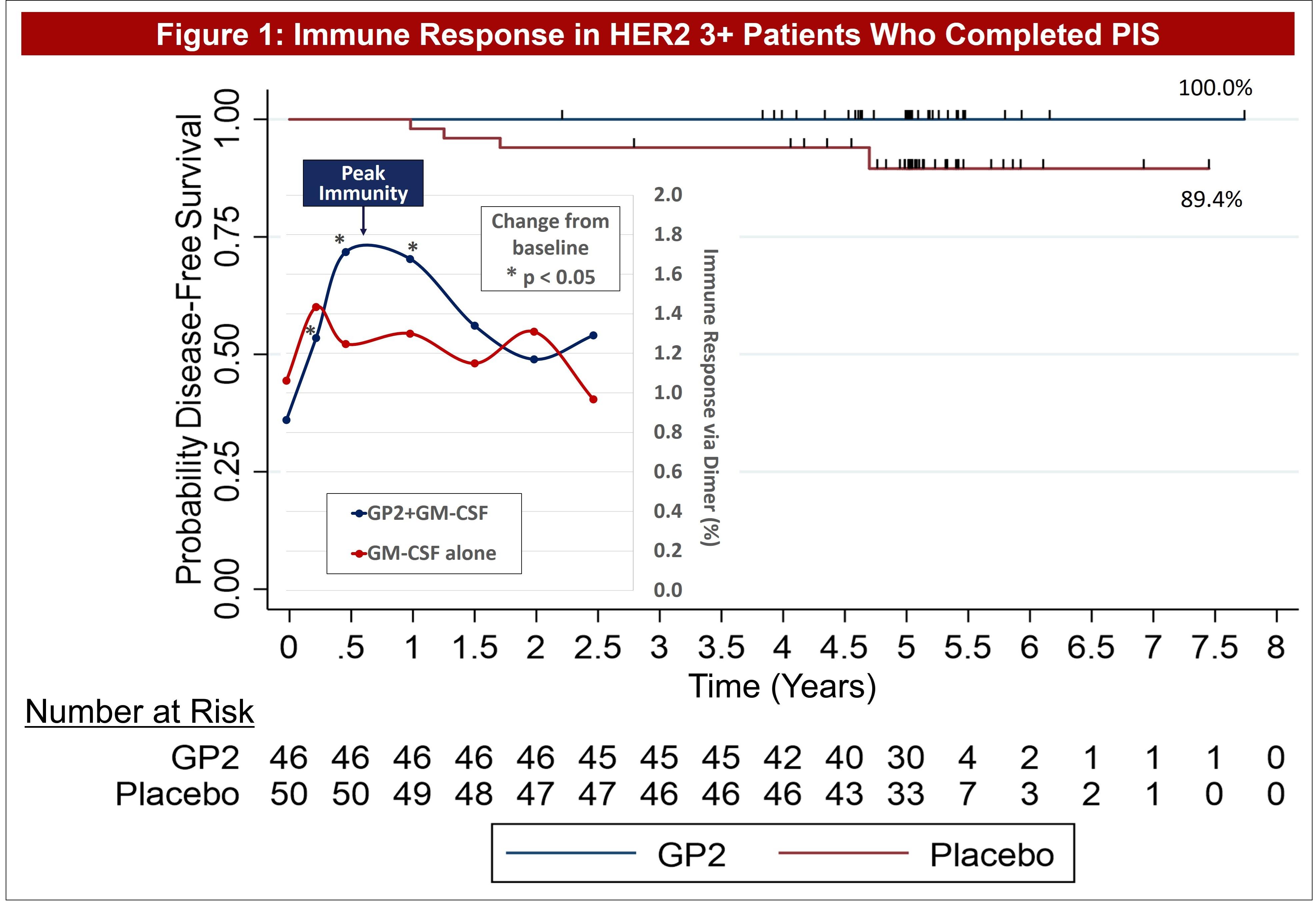
| ● | Figure 1 of the poster shows that GP2 immunity peaked at 6 months in HER2 3+ patients after they completed their first 6 immunizations, as measured by the Dimer Binding Assay. The data also shows that for the 2.5 years that the immune response was measured, the immunity was sustained and remained above baseline, resulting in 100% disease free survival (0% recurrence rate) over 5 years. In the placebo arm, the immune response was not as robust, resulting in 89% disease free survival (11% recurrence rate). | |
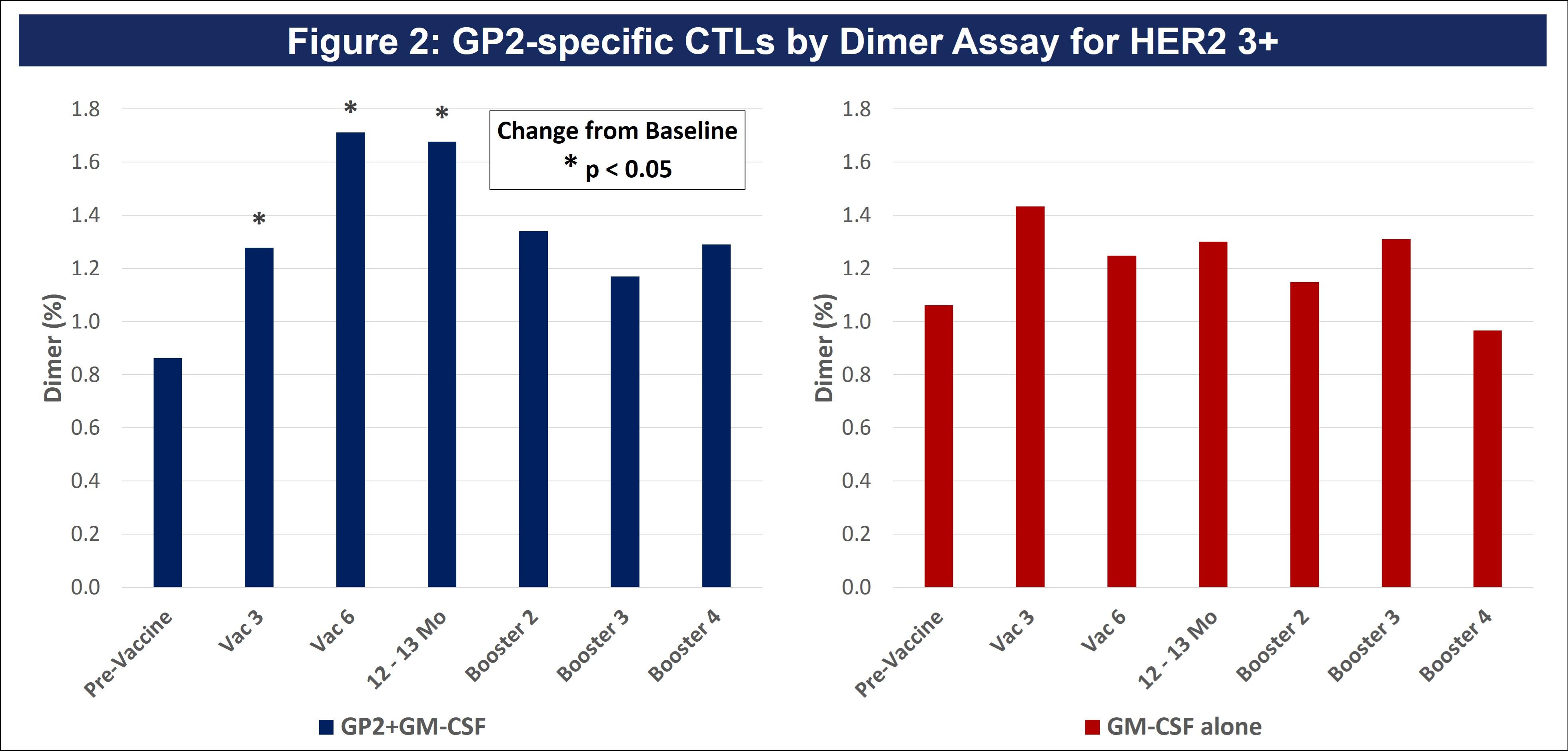
| ● | Figure 2 of the poster shows the same Dimer Binding Assay data for HER2 3+ patients as in Figure 1, where the GP2 treated patients showed statistically significant dimer readings versus baseline at 3, 6, and 12-13 months. | |
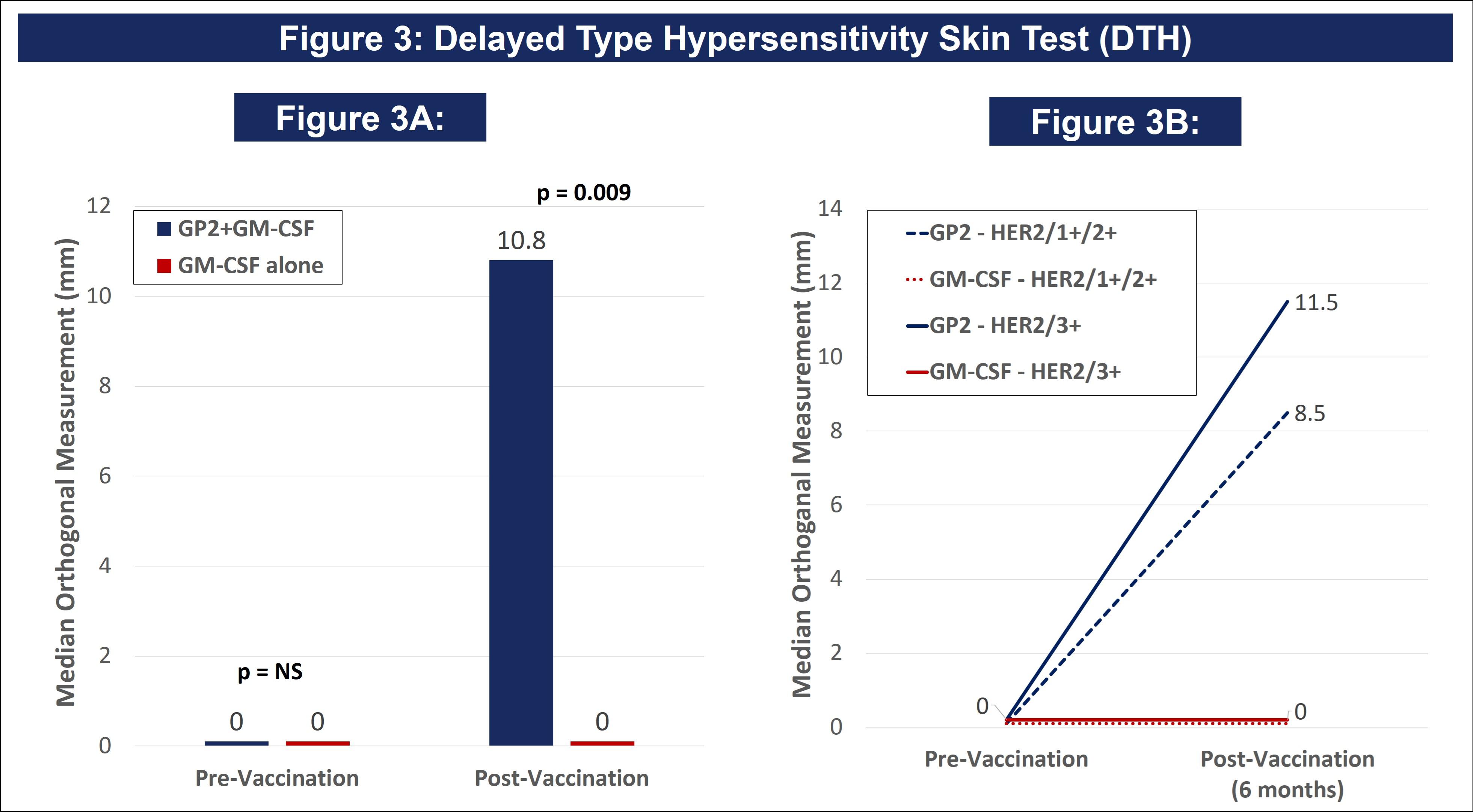
| ● | Figure 3 of the poster shows that after completion of the 6th immunization after 6 months, GP2 treated patients showed a robust immune response using the DTH skin test, while the placebo did not (p = 0.009). Within GP2-treated patients, the change from baseline after 6 months was a median of 4.8 mm (mean of 11.6 mm), which was a statistically significant increase over baseline (p < 0.0001). This DTH data supports the Dimer Binding Assay data that shows a peak immune response after 6 months. |
STAFFORD, Texas—(Business Wire)— Greenwich LifeSciences, Inc. (Nasdaq: GLSI) (the “Company”), a clinical-stage biopharmaceutical company focused on the development of GP2, an immunotherapy to prevent breast cancer recurrences in patients who have previously undergone surgery, today presented a poster of the final 5 year GP2 Phase IIb clinical trial immune response data at the 2021 AACR Annual Meeting. Immune response is the primary mechanism of action for GP2 and is critical to developing dosing and booster treatment strategies that are designed to achieve and sustain peak immunity, as well as to prevent metastatic breast cancer recurrences.
It has been previously reported that the completion of the GP2+GM-CSF Primary Immunization Series (PIS) reduced recurrence rates to 0% over a 5 year follow-up period in HER2 3+ patients who had received a standard course of trastuzumab after surgery. The abstract and poster present the final immune response results over the 5 year follow-up period, assessing peak immunity compared to baseline and between patients treated with GP2+GM-CSF versus GM-CSF alone, including by HER2 status.
Summary of the Final 5 Year Immune Response Data as Previously Presented:
| ● | Potent immune response data supports the previously reported clinical outcome of 0% metastatic breast cancer recurrences over 5 years of follow up, if a patient completes the Primary Immunization Series over the first 6 months of GP2 treatment. | |
| ● | Statistically significant peak immunity was reached after 6 months of GP2 treatment as measured in both the Dimer Binding Assay and the DTH skin test. | |
| ● | HER2 3+ population immune response was similar to the HER2 1-2+ population immune response, suggesting the potential to treat the HER2 1-2+ population (including triple negative breast cancer) with GP2 immunotherapy in combination with trastuzumab (Herceptin) based products and other clinically active agents. | |
| ● | Broad based immune response suggests that GP2 immunotherapy and Herceptin based products may also have the potential to treat other HER2 1-3+ expressing cancers. |
Dr. Thompson commented, “The analysis of the immune response data in the Phase IIb trial provides mechanistic confirmation of treatment effect correlated with the clinical response previously reported. GP2 treated patients, independent of their HER2 status, experienced a potent immune response to GP2, far greater than patients treated with placebo. In addition, this data has provided us with insight that will guide the upcoming Phase III trial. We believe that monitoring immune response will be an important aspect of the Phase III trial.”
Excerpts from the AACR Poster CT183:
Title: Final five year median follow-up data from a prospective, randomized, placebo-controlled, single-blinded, multicenter, phase IIb study evaluating a time series of immune responses using HER2/neu peptide GP2 + GM-CSF vs. GM-CSF alone after adjuvant trastuzumab in HER2 positive women with operable breast cancer
Each GP2 treated patient was scheduled to receive 6 intradermal injections with GP2+GM-CSF over the first 6 months of treatment as part of the Primary Immunization Series and 4 boosters every 6 months thereafter. Placebo patients received intradermal injections with GM-CSF alone.
Immune responses to GP2 were measured over time using a CD8 T cell dimer binding assay (Dimer Binding Assay) and delayed-type-hypersensitivity (DTH) skin tests. The Dimer Binding Assay detects the percentage of GP2 specific killer T cells that can kill recurring cancer cells. The DTH skin test measures the diameter of the skin immune response to GP2 in millimeters 48-72 hours after injection of GP2 without GM-CSF.
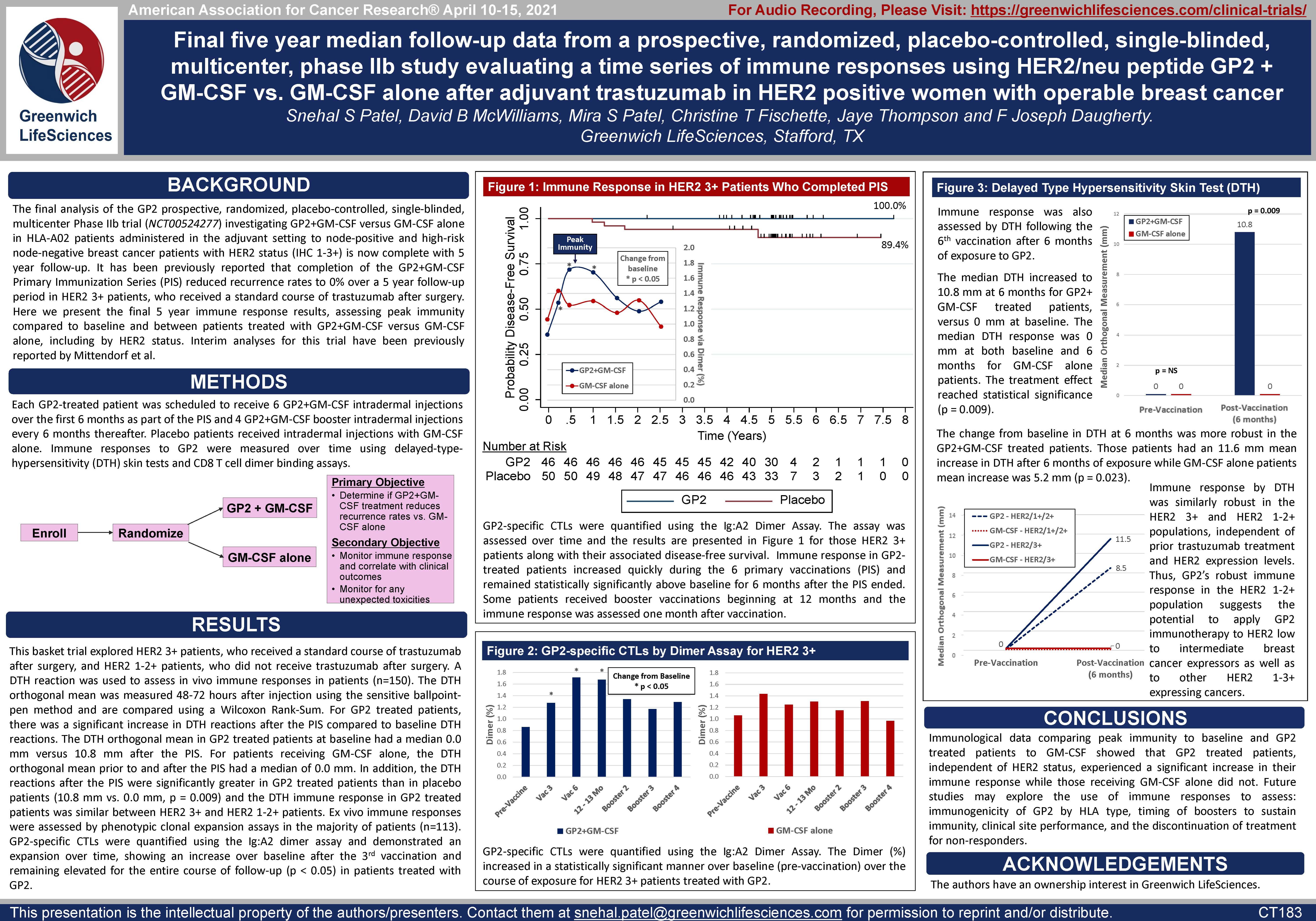
Figure 1 of the poster shows that GP2 immunity peaked at 6 months in HER2 3+ patients after they completed their first 6 immunizations, as measured by the Dimer Binding Assay. The data also shows that for the 2.5 years that the immune response was measured, the immunity was sustained and remained above baseline, resulting in 100% disease free survival (0% recurrence rate) over 5 years. In the placebo arm, the immune response was not as robust, resulting in 89% disease free survival (11% recurrence rate). Immune response in GP2-treated patients increased quickly during the Primary Immunization Series and remained statistically significantly above baseline for 6 months after the completion of the Primary Immunization Series. Some patients received boosters beginning at 12 months and the immune response was assessed one month after the receiving the booster.
Dimer Binding Assay: The Dimer Binding Assay detects the percentage of GP2 specific killer T cells that can kill recurring cancer cells. Ex vivo immune response was assessed over 2.5 years with blood draws at baseline, then after the 3rd and 6th immunizations in the Primary Immunization Series, and then after each booster. Immune responses were assessed by phenotypic clonal expansion assays in the majority of patients (n=113). GP2-specific CTLs were quantified in patients treated with GP2 using the Ig:A2 Dimer Assay and demonstrated an expansion over time, showing an increase over baseline after the 3rd immunization and remaining elevated for the entire course of follow-up.
Figure 2 of the poster shows the same Dimer Binding Assay data for HER2 3+ patients as in Figure 1, where the GP2 treated patients showed statistically significant dimer readings versus baseline (pre-vaccination) at 3, 6, and 12-13 months.
DTH Skin Test: The DTH skin test measures the diameter of the skin immune response to GP2 in millimeters, 48-72 hours after intradermal injection of GP2 without GM-CSF. A DTH reaction was used to assess in vivo immune responses in patients (n=150). The DTH orthogonal mean of the skin wheal was measured 48-72 hours after injection using the sensitive ballpoint-pen method and is compared using a Wilcoxon Rank-Sum. For GP2 treated patients, there was a significant increase in DTH reactions after the PIS compared to baseline DTH reactions.
Figure 3A shows that after completion of the 6th immunization after 6 months, GP2 treated patients showed a robust immune response using the DTH skin test, while the placebo did not (p = 0.009). Within GP2 treated patients, the change from baseline after 6 months was a median of 4.8 mm (mean of 11.6 mm), which was a statistically significant increase over baseline (p < 0.0001). The change from baseline in DTH at 6 months was more robust in the GP2 treated patients. Those patients had an 11.6 mm mean increase in DTH after 6 months of exposure while patients treated with GM-CSF alone had a 5.2 mm mean increase (p = 0.023). This DTH data supports the Dimer Binding Assay data that shows a peak immune response after 6 months.
Figure 3B shows that the DTH immune response for GP2 treated patients was similarly robust in HER2 3+ patients and HER2 1-2+ patients, independent of prior trastuzumab treatment and HER2 expression levels. Thus, GP2’s robust immune response in the HER2 1-2+ population suggests the potential to apply GP2 immunotherapy to HER2 low to intermediate expressing breast cancers, as well as to other HER2 1-3+ expressing cancers.
AACR Abstract CT183:
Title: Final five year median follow-up data from a prospective, randomized, placebo-controlled, single-blinded, multicenter, phase IIb study evaluating a time series of immune responses using HER2/neu peptide GP2 + GM-CSF vs. GM-CSF alone after adjuvant trastuzumab in HER2 positive women with operable breast cancer
Snehal S Patel, David B McWilliams, Mira S Patel, Christine T Fischette, Jaye Thompson and F Joseph Daugherty.
Greenwich LifeSciences, Stafford, TX
Background: The final analysis of the GP2 prospective, randomized, placebo-controlled, single-blinded, multicenter Phase IIb trial (NCT00524277) investigating GP2+GM-CSF versus GM-CSF alone in HLA-A02 patients administered in the adjuvant setting to node-positive and high-risk node-negative breast cancer patients with HER2 status (IHC 1-3+) is now complete with 5 year follow-up. It has been previously reported that completion of the GP2+GM-CSF Primary Immunization Series (PIS) reduced recurrence rates to 0% over a 5 year follow-up period in HER2 3+ patients, who received a standard course of trastuzumab after surgery. Here we present the final immune response results, assessing peak immunity compared to baseline and between GP2 treated patients versus placebo, including by HER2 status. Interim analyses for this trial have been previously reported by Mittendorf et al.
Methods: Each GP2-treated patient was scheduled to receive 6 GP2+GM-CSF intradermal injections over the first 6 months as part of the PIS and 4 GP2+GM-CSF booster intradermal injections every 6 months thereafter. Placebo patients received GM-CSF only intradermal injections. Immune responses to GP2 were measured over time using delayed-type-hypersensitivity (DTH) skin tests and CD8 Tcell dimer binding assays.
Results: This basket trial explored HER2 3+ patients, who received a standard course of trastuzumab after surgery, and HER2 1-2+ patients, who did not receive trastuzumab after surgery. A DTH reaction was used to assess in vivo immune responses in patients (n=145). The DTH orthogonal mean was measured 48-72 hours after injection using the sensitive ballpoint-pen method and are compared using a Wilcoxon Rank-Sum. For GP2 treated patients, there was a significant increase in DTH reactions after the PIS compared to baseline DTH reactions. The DTH orthogonal mean in GP2 treated patients at baseline had a median 0.0mm versus 10.8mm after the PIS. For patients receiving GM-CSF alone, the DTH orthogonal mean prior to and after the PIS had a median of 0.0mm. In addition, the DTH reactions after the PIS were significantly greater in GP2 treated patients than in placebo patients (10.8mm vs. 0.0mm, p=0.009) and the DTH immune response in GP2 treated patients was similar between HER2 3+ and HER2 1-2+ patients. Ex vivo immune responses were assessed by phenotypic clonal expansion assays in the majority of patients (n=114). GP2-specific CTLs were quantified using the Ig:A2 dimer assay and demonstrated a gradual expansion over time reaching statistical significance approximately 6 months after the PIS compared to baseline in the GP2 treated patients (n=53, p=0.010) but not in the control patients (n=39, p=0.165).
Conclusions: Immunological data comparing peak immunity to baseline and GP2 treated patients to placebo showed that GP2 treated patients, independent of HER2 status, experienced a significant increase in their immune response while those receiving GM-CSF only did not. Future studies may explore the use of immune responses to assess: immunogenicity of GP2 by HLA type, timing of boosters to sustain immunity, clinical site performance, and the discontinuation of treatment for non-responders.
About the AACR Annual Meeting 2021
The AACR is the first and largest cancer research organization dedicated to accelerating the conquest of cancer and has more than 48,000 members residing in 127 countries and territories. The AACR Annual Meeting program covers the latest discoveries across the spectrum of cancer research — from population science and prevention; to cancer biology, translational, and clinical studies; to survivorship and advocacy — and highlights the work of the best minds in research and medicine from institutions all over the world.
About Breast Cancer and HER2/neu Positivity
One in eight U.S. women will develop invasive breast cancer over her lifetime, with approximately 266,000 new breast cancer patients and 3.1 million breast cancer survivors in 2018. HER2/neu (human epidermal growth factor receptor 2) protein is a cell surface receptor protein that is expressed in a variety of common cancers, including in 75% of breast cancers at low (1+), intermediate (2+), and high (3+ or over-expressor) levels.
About Greenwich LifeSciences, Inc.
Greenwich LifeSciences is a clinical-stage biopharmaceutical company focused on the development of GP2, an immunotherapy to prevent breast cancer recurrences in patients who have previously undergone surgery. GP2 is a 9 amino acid transmembrane peptide of the HER2/neu protein. In a randomized, single-blinded, placebo-controlled, multi-center (16 sites led by MD Anderson Cancer Center) Phase IIb clinical trial, no recurrences were observed in the HER2/neu 3+ adjuvant setting after median 5 years of follow-up, if the patient received the 6 primary intradermal injections over the first 6 months (p = 0.0338). Of the 138 patients that have been treated with GP2 to date over 4 clinical trials, GP2 treatment was well tolerated and no serious adverse events were observed related to GP2 immunotherapy. Greenwich LifeSciences is planning to commence a Phase III clinical trial using a similar treatment regime as the Phase IIb clinical trial. For more information on Greenwich LifeSciences, please visit the Company’s website at www.greenwichlifesciences.com and follow the Company’s Twitter at https://twitter.com/GreenwichLS.
Forward-Looking Statement Disclaimer
Statements in this press release contain “forward-looking statements” that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release may be identified by the use of words such as “anticipate,” “believe,” “contemplate,” “could,” “estimate,” “expect,” “intend,” “seek,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “target,” “aim,” “should,” “will,” “would,” or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements are based on Greenwich LifeSciences Inc.’s current expectations and are subject to inherent uncertainties, risks and assumptions that are difficult to predict, including statements regarding the intended use of net proceeds from the public offering; consequently, actual results may differ materially from those expressed or implied by such forward-looking statements. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully in the section titled “Risk Factors” in the final prospectus related to the public offering filed with the SEC. Forward-looking statements contained in this announcement are made as of this date, and Greenwich LifeSciences, Inc. undertakes no duty to update such information except as required under applicable law.
Company Contact
Snehal Patel
Investor Relations
(832) 819-3232
info@greenwichlifesciences.com
Investor & Public Relations Contact for Greenwich LifeSciences
Dave Gentry
RedChip Companies Inc.
Office: 1-800-RED CHIP (733 2447)
Cell: (407) 491-4498
dave@redchip.com